What is the pH of water?
When we think of water, we often envision a clear, tasteless, and neutral liquid, essential for life. This neutrality is not just a sensory perception but is rooted in the chemical nature of water, specifically its pH. So, what exactly is the pH of water?
The pH scale measures the acidity or alkalinity of a solution based on its hydrogen ion concentration. The pH scale ranges from 0 to 14, with a pH of 7 considered neutral, values below 7 are acidic, and those above 7 are alkaline. Pure water, in its intrinsic state and at room temperature, has a pH of 7, making it neutral. This neutrality arises from water’s unique molecular structure. Each water molecule can dissociate into a hydrogen ion (H+) and a hydroxide ion (OH-). In pure water, the concentrations of these ions are equal, resulting in a balanced, neutral pH.
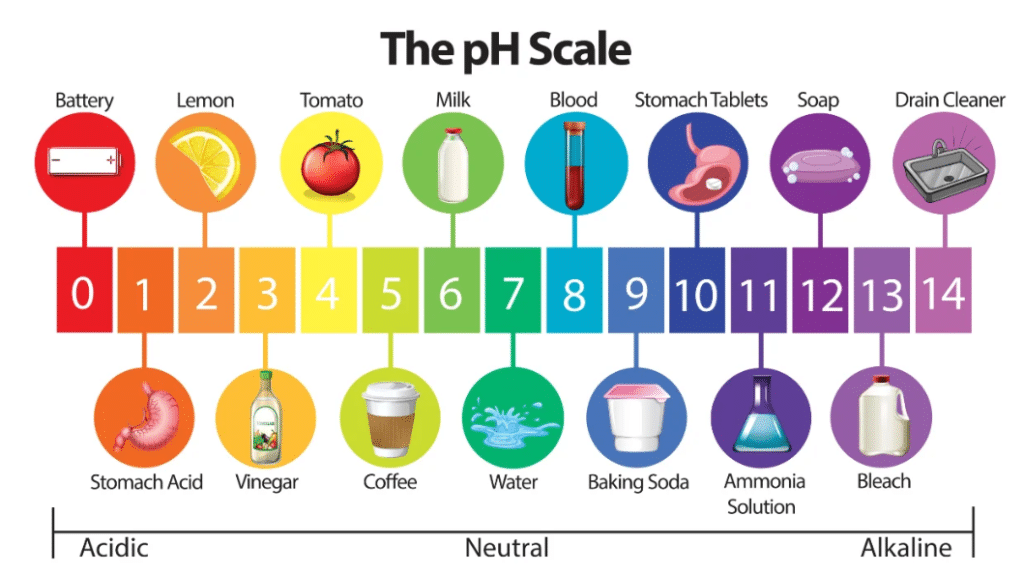
| Substance | pH Value |
|---|---|
| Battery Acid | 0 |
| Vinegar | 2.5-3.5 |
| Lemon Juice | 2-3 |
| Pure Water | 7 |
| Blood | 7.35-7.45 |
| Seawater | 8 |
| Bleach | 12 |
PH Comparison with Common Liquids
However, water’s pH can be influenced by various factors. When water interacts with substances in the environment, its pH can shift. For instance, rainwater absorbs carbon dioxide from the atmosphere, forming carbonic acid. This makes rainwater slightly acidic, typically giving it a pH of around 5.6. Similarly, water sources near industrial areas or agricultural runoffs might have altered pH levels due to pollutants or chemicals.
While pure water has a neutral pH of 7, various natural and man-made factors can influence this value.
Introduction to pH and its Importance
The concept of pH dates back to the early 20th century when it was introduced by the Danish chemist Søren Peder Lauritz Sørensen. The term “pH” stands for “potential of hydrogen,” and it measures the hydrogen ion concentration in a solution. In simpler terms, pH indicates the acidity or alkalinity of a substance.
But why is this measure so crucial? pH plays a fundamental role in various chemical reactions. It can influence the solubility of minerals, the taste of food and beverages, and the effectiveness of many chemical processes.
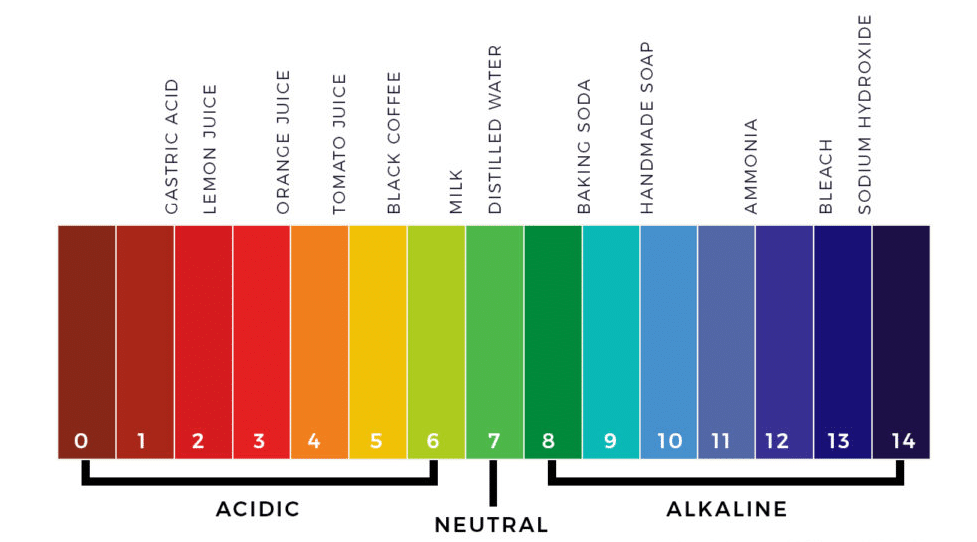
In the human body, maintaining a stable pH is essential for various metabolic processes. Enzymes, the biological catalysts that facilitate chemical reactions in our cells, often have a specific pH range in which they function optimally. For instance, the normal pH of drinking water is around 7, which is neutral and safe for consumption.
Deviations from this range can hinder their activity, leading to metabolic imbalances. Similarly, the pH of the environment can influence the health and diversity of aquatic ecosystems. Certain fish species, for instance, thrive only in specific pH ranges. A shift in the pH can disrupt their reproductive cycles, leading to population declines. Thus, from the microscopic cellular level to vast aquatic ecosystems, pH plays a pivotal role in maintaining the balance of life.
Safe Range for Drinking Water pH Levels
Water is a universal solvent, capable of dissolving a wide range of substances. As it flows through the environment, it can pick up minerals, organic matter, and pollutants, all of which can influence its pH. For drinking water, the World Health Organization and the Environmental Protection Agency recommend a pH range of 6.5 to 8.5. But why this specific range?
Water with a pH below 6.5 tends to be acidic. Acidic water can corrode metal pipes, leading to the leaching of metals like copper and lead into the water. Consuming water with elevated metal levels can have adverse health effects. When drinking water pH falls outside the safe range, it can lead to corrosion, contamination, and the need for professional testing and water treatment. On the other hand, water with a pH above 8.5 is alkaline. Alkaline water can cause scale buildup in pipes and appliances, leading to plumbing issues and increased maintenance costs. Moreover, drastic deviations from the neutral pH can influence the taste and odor of water.
Acidic water might have a sour taste, while alkaline water can taste bitter or soda-like. Thus, for both health and aesthetic reasons, maintaining water within the recommended pH range is essential.

Importance of pH in Water Quality
Water quality is a multifaceted concept, encompassing various physical, chemical, and biological parameters. Among these, pH stands out as a fundamental measure. The importance of pH in drinking water quality cannot be overstated. At a basic level, pH can directly influence the sensory properties of water, such as its taste and clarity.
For instance, water that’s too acidic or too alkaline might not be palatable, deterring people from consuming adequate amounts. Beyond sensory properties, pH plays a pivotal role in determining the solubility and toxicity of various chemical substances in water.
Many metals, for instance, become more soluble in acidic conditions. This means that acidic waters can carry higher concentrations of metals like lead, copper, and zinc, posing health risks to consumers. On the other hand, alkaline waters can lead to scale formation, affecting both household plumbing and industrial processes. Furthermore, pH can influence the efficacy of water disinfection. Chlorine, a common disinfectant, is more effective at an optimal pH range.

Deviations from this range can reduce its disinfection capability, potentially allowing harmful microbes to thrive. In aquatic ecosystems, pH can influence the health and diversity of species. Many aquatic organisms, from tiny plankton to large fish, have specific pH ranges where they thrive. Changes in pH can disrupt their life cycles, leading to ecological imbalances. Given these wide-ranging implications, monitoring and managing pH is a cornerstone of water quality management.
Acidic vs. Alkaline Water
The terms “acidic” and “alkaline” are often used to describe the pH spectrum. But what do they truly mean in the context of water? Acidic water has a pH value below 7, indicating a higher concentration of hydrogen ions (H+). This can arise due to natural processes, such as the dissolution of carbon dioxide forming carbonic acid, or through human activities, like acid rain resulting from industrial emissions. Acidic waters can pose several challenges. They can corrode metal infrastructure, leach harmful metals into the water, and harm aquatic life.
Alkaline water, with a pH value above 7, indicates a higher concentration of hydroxide ions (OH-). Alkalinity can result from the dissolution of minerals like limestone or from certain industrial discharges. While alkaline waters are often touted for potential health benefits, excessive alkalinity can pose challenges too. Consuming alkaline water may provide minerals and electrolytes, but it is important to ensure the pH is within a safe range. It can lead to scale formation, affect the taste of water, and reduce the efficiency of certain water treatments. Drinking alkaline water may benefit some individuals, but it is crucial to monitor the pH to avoid potential health risks. Understanding the sources and implications of both acidic and alkaline waters is crucial for effective water management.

Risks and consequences of unusual pH levels in water
Marine Life: One of the most significant consequences of unusual pH levels in water is the acidification of oceans and lakes. When the pH of water drops below 7, it becomes more acidic, which can have devastating effects on marine life. Many aquatic species have specific pH requirements for survival and reproduction.
Changes in pH can disrupt the chemical balance of the water and make it difficult for these species to thrive. For example, low pH levels can cause fish to become disoriented and affect their growth and reproductive abilities. Acidification can cause shellfish and coral to dissolve, disrupt the food chain, and ultimately harm commercial fishing and tourism industries.
Human Life: Water with a low pH can be corrosive and cause lead and other metals to leach into the water, which can be harmful to human health. Water with a high pH can have a bitter taste and make it difficult for the body to absorb essential minerals. Additionally, changes in pH can affect the effectiveness of water treatment methods, leading to potential health risks. The U.S. Environmental Protection Agency (EPA) monitors public drinking water quality by measuring pH levels to ensure safety and quality.

While the ideal pH level of drinking water should be 6 to 8.5, the human body maintains pH equilibrium on a constant basis and will not be affected by water consumption. For example, our stomachs have a naturally low pH level of 2 which is a beneficial acidity that helps us with food digestion.
Natural and Man-made Factors Influencing pH
Water, as it moves through the environment, interacts with various elements, both natural and man-made, that can influence its pH. Naturally occurring factors play a significant role. For instance, the geology of an area can directly impact the pH of surface and groundwater. Regions with limestone or chalk bedrock tend to have more alkaline waters due to the dissolution of carbonate minerals. Conversely, areas with peat or certain types of granite can produce more acidic waters.
Photosynthesis, a process carried out by plants, can also influence pH. As plants absorb carbon dioxide (a weak acid) from the water, they can cause a rise in pH, making the water more alkaline. Rain, especially in industrialized areas, can be acidic due to the presence of sulfuric and nitric acids formed from industrial emissions. This “acid rain” can lower the pH of water bodies, affecting aquatic life.
On the other hand, human activities have introduced a range of pH influencers. Industrial discharges, especially from industries like mining or manufacturing, can introduce acids or bases into water systems. Agriculture, a cornerstone of human civilization, can also impact pH.
The use of fertilizers, lime, and other chemicals can alter the pH of runoff water, which eventually finds its way into rivers and lakes. Urban runoff, from roads and buildings, can carry a cocktail of chemicals, from acidic pollutants to alkaline concrete dust, influencing the pH of urban waterways. Tap water, often treated with disinfectants like chlorine, also requires monitoring to maintain safe pH levels. Given the myriad of factors at play, managing and maintaining optimal pH levels in water bodies is a complex, yet crucial task.
Conducting a pH Test
Understanding the importance of pH is one thing, but how do we measure it in practice? The process, while rooted in complex chemistry, is relatively straightforward in application. Traditional methods, like the use of litmus paper, offer a quick and visual representation of pH. When dipped in a solution, the paper changes color based on the solution’s acidity or alkalinity. While this method provides a general indication, it lacks precision. For more accurate readings, electronic pH meters are employed.
These devices, which range from handheld probes to sophisticated laboratory equipment, measure the electrical potential in a solution. Since this potential is directly related to the concentration of hydrogen ions, it provides a precise pH reading. To ensure accuracy, pH meters are regularly calibrated using solutions of known pH. For those keen on understanding their water’s pH, whether for gardening, aquariums, or personal consumption, a range of commercial testing kits are available. Regular testing is especially crucial for industries and municipalities to ensure water safety and compliance with environmental standards.
Remedies for Off pH Levels
So, what happens if the pH of your water isn’t optimal? Thankfully, there are remedies available. For waters that are too acidic, neutralizing filters or chemical feed pumps can be used. These systems introduce a base, like calcium carbonate or soda ash, to raise the pH. For waters that are too alkaline, acid injection systems can be employed.
These systems introduce a mild acid, like vinegar or citric acid, to lower the pH. It’s worth noting that while adjusting pH can remedy certain issues, it’s essential to consider the broader water chemistry. For instance, raising the pH can lead to increased scaling potential, while lowering it can increase corrosion potential. As such, pH adjustment is often done in conjunction with other water treatments to ensure overall water quality.

Conclusion
In the vast realm of water science, pH stands out as a fundamental yet profound measure. It influences everything from the taste of our morning coffee to the health of our aquatic ecosystems. As we continue to shape our environment, through urbanization, agriculture, and industry, understanding and managing pH becomes ever more crucial. By ensuring our waters remain within optimal pH ranges, we not only safeguard our health but also protect the delicate balance of our planet’s ecosystems.
